Chemical properties of water
Keywords
Molecule Electron Covalent bond Solvent Cappilary action Dipole momentMolecular Structure
Water’s molecular structure is simple yet very important. It consists of two hydrogen atoms bonded to one oxygen atom, forming the chemical formula H2O. The oxygen atom attracts electrons more strongly than hydrogen atoms. As a result, the electrons are pulled closer to the oxygen atom, creating a partial negative charge around oxygen and partial positive charges around the hydrogen atoms. This difference in charge leads to a polar covalent bond, where the electrons are unevenly shared. What is the effect of this on macro scale?
Did not understand it yet? Do not worry we will get back to this. For now remember that the water molecule has a bent shape
The arrangement of the atoms in a water molecule is bent rather than linear. This bent shape amplifies the molecule’s polarity, creating a dipole moment. This polarity is the reason for many of water’s unique characteristics.
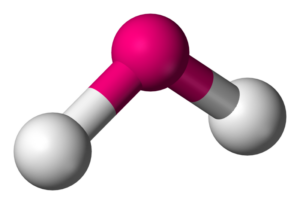
Bent shape
 /*! elementor – v3.19.0 – 29-01-2024 */
.elementor-column .elementor-spacer-inner{height:var(–spacer-size)}.e-con{–container-widget-width:100%}.e-con-inner>.elementor-widget-spacer,.e-con>.elementor-widget-spacer{width:var(–container-widget-width,var(–spacer-size));–align-self:var(–container-widget-align-self,initial);–flex-shrink:0}.e-con-inner>.elementor-widget-spacer>.elementor-widget-container,.e-con>.elementor-widget-spacer>.elementor-widget-container{height:100%;width:100%}.e-con-inner>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer,.e-con>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer{height:100%}.e-con-inner>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer>.elementor-spacer-inner,.e-con>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer>.elementor-spacer-inner{height:var(–container-widget-height,var(–spacer-size))}.e-con-inner>.elementor-widget-spacer.elementor-widget-empty,.e-con>.elementor-widget-spacer.elementor-widget-empty{position:relative;min-height:22px;min-width:22px}.e-con-inner>.elementor-widget-spacer.elementor-widget-empty .elementor-widget-empty-icon,.e-con>.elementor-widget-spacer.elementor-widget-empty .elementor-widget-empty-icon{position:absolute;top:0;bottom:0;left:0;right:0;margin:auto;padding:0;width:22px;height:22px}
/*! elementor – v3.19.0 – 29-01-2024 */
.elementor-column .elementor-spacer-inner{height:var(–spacer-size)}.e-con{–container-widget-width:100%}.e-con-inner>.elementor-widget-spacer,.e-con>.elementor-widget-spacer{width:var(–container-widget-width,var(–spacer-size));–align-self:var(–container-widget-align-self,initial);–flex-shrink:0}.e-con-inner>.elementor-widget-spacer>.elementor-widget-container,.e-con>.elementor-widget-spacer>.elementor-widget-container{height:100%;width:100%}.e-con-inner>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer,.e-con>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer{height:100%}.e-con-inner>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer>.elementor-spacer-inner,.e-con>.elementor-widget-spacer>.elementor-widget-container>.elementor-spacer>.elementor-spacer-inner{height:var(–container-widget-height,var(–spacer-size))}.e-con-inner>.elementor-widget-spacer.elementor-widget-empty,.e-con>.elementor-widget-spacer.elementor-widget-empty{position:relative;min-height:22px;min-width:22px}.e-con-inner>.elementor-widget-spacer.elementor-widget-empty .elementor-widget-empty-icon,.e-con>.elementor-widget-spacer.elementor-widget-empty .elementor-widget-empty-icon{position:absolute;top:0;bottom:0;left:0;right:0;margin:auto;padding:0;width:22px;height:22px}
Linear shape
The effects of water’s molecular structure are as follows:
/*! elementor-pro – v3.19.0 – 29-01-2024 */ .elementor-slides .swiper-slide-bg{background-size:cover;background-position:50%;background-repeat:no-repeat;min-width:100%;min-height:100%}.elementor-slides .swiper-slide-inner{background-repeat:no-repeat;background-position:50%;position:absolute;top:0;left:0;bottom:0;right:0;padding:50px;margin:auto}.elementor-slides .swiper-slide-inner,.elementor-slides .swiper-slide-inner:hover{color:#fff;display:flex}.elementor-slides .swiper-slide-inner .elementor-background-overlay{position:absolute;z-index:0;top:0;bottom:0;left:0;right:0}.elementor-slides .swiper-slide-inner .elementor-slide-content{position:relative;z-index:1;width:100%}.elementor-slides .swiper-slide-inner .elementor-slide-heading{font-size:35px;font-weight:700;line-height:1}.elementor-slides .swiper-slide-inner .elementor-slide-description{font-size:17px;line-height:1.4}.elementor-slides .swiper-slide-inner .elementor-slide-description:not(:last-child),.elementor-slides .swiper-slide-inner .elementor-slide-heading:not(:last-child){margin-bottom:30px}.elementor-slides .swiper-slide-inner .elementor-slide-button{border:2px solid #fff;color:#fff;background:transparent;display:inline-block}.elementor-slides .swiper-slide-inner .elementor-slide-button,.elementor-slides .swiper-slide-inner .elementor-slide-button:hover{background:transparent;color:inherit;text-decoration:none}.elementor–v-position-top .swiper-slide-inner{align-items:flex-start}.elementor–v-position-bottom .swiper-slide-inner{align-items:flex-end}.elementor–v-position-middle .swiper-slide-inner{align-items:center}.elementor–h-position-left .swiper-slide-inner{justify-content:flex-start}.elementor–h-position-right .swiper-slide-inner{justify-content:flex-end}.elementor–h-position-center .swiper-slide-inner{justify-content:center}body.rtl .elementor-widget-slides .elementor-swiper-button-next{left:10px;right:auto}body.rtl .elementor-widget-slides .elementor-swiper-button-prev{right:10px;left:auto}.elementor-slides-wrapper div:not(.swiper-slide)>.swiper-slide-inner{display:none}@media (max-width:767px){.elementor-slides .swiper-slide-inner{padding:30px}.elementor-slides .swiper-slide-inner .elementor-slide-heading{font-size:23px;line-height:1;margin-bottom:15px}.elementor-slides .swiper-slide-inner .elementor-slide-description{font-size:13px;line-height:1.4;margin-bottom:15px}} Universal SolventSolvent Properties: The polar nature of water makes it an excellent solvent. This means that other particles will be absorbed into the fluid. It can dissolve a wide range of substances, facilitating essential biological and chemical processes. How does the polar property make it a solvent?Read moreHydrogen BondingHydrogen Bonding: The partial charges on water molecules allow them to form hydrogen bonds with other water molecules. This gives water its cohesion, surface tension, and high heat capacity. Hydrogen bonding is also crucial in biological systems.Read moreDensity AnomalyDensity Anomaly: Water is densest at 4 degrees Celsius, and its density decreases as it freezes. This anomaly allows ice to float on water, insulating the liquid below and supporting aquatic life during cold seasons.Click Here Previous slide Next slideLet’s take a closer look at the hydrogen bond
https://www.youtube.com/watch?v=m-v5G8C70pc
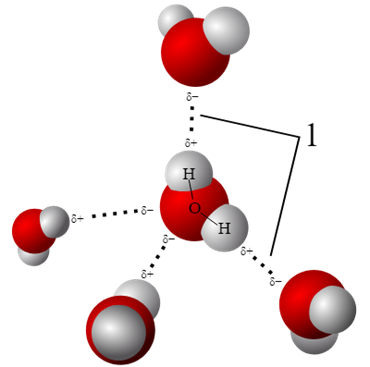
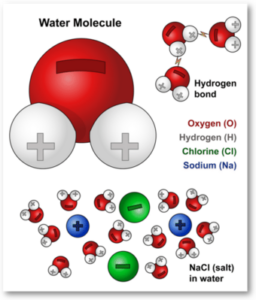
Remember: The hydrogen bond is a result of the polar charges as seen on the picture. The opposite charged sides of the molecules attract eachother. We will see that this force is enabling the surface pressure on water later on.
- High Heat Capacity: Water can absorb and retain a significant amount of heat without a substantial rise in temperature. This property helps regulate Earth’s climate and moderates temperature changes in living organisms.
- Adhesion and Cohesion: Water molecules are attracted to each other (cohesion) and to other substances (adhesion). These properties contribute to capillary action, allowing water to move against gravity in plants and small tubes.
Understanding the molecular structure of water provides insights into its vital role in various natural processes and living organisms. This simple arrangement of atoms gives rise to the complexity and beauty of water’s behavior in our world.
Let’s revisit the concept of water as a solvent
https://www.youtube.com/watch?v=2QSDu4DMr7A2. Universal Solvent
Water’s ability to act as a universal solvent is a consequence of its polar nature. The oxygen atom’s electronegativity pulls electrons towards itself, creating a partial negative charge, while the hydrogen atoms carry partial positive charges. This polarity enables water to break down and dissolve a wide array of substances, facilitating chemical reactions necessary for life.
- Bloodcells
- Plants
- Organs
3. Polarity
The polarity of water not only makes it an excellent solvent but also results in surface tension—a phenomenon that allows small organisms like water striders to “walk” on the water’s surface. Additionally, the cohesive forces between water molecules create a high level of tension, giving rise to capillary action, which is crucial for water transport in plants.
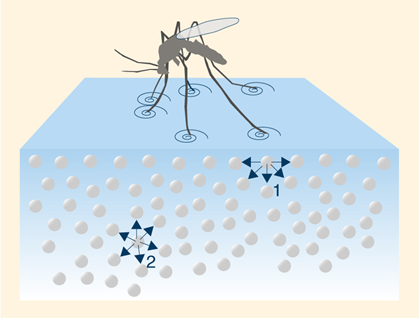
4. Vaporization
Water’s high heat of vaporization is a remarkable property that plays an important role in moderating Earth’s climate. When water evaporates, it absorbs heat energy from its surroundings, cooling the environment. Conversely, when water vapor condenses, it releases heat, warming the surrounding air. This process is integral to the water cycle and climate regulation. These characteristics of water make it possible to fulfil different functions in nature and impact our daily lives. By understanding these concepts, we will be able to connect the current problems that humanity is facing around the world to the scientific reasoning behind it. We often cannot change the chemical properties of nature as a Wavemaker. However, we can change the way we interact with it. This in turn will have a positive effect. Let us continue with some more physical properties and explore what we can do!