Physical properties of water
In the world around us, water isn’t just something we drink or splash around—it’s a real gamechanger. Imagine a quiet stream, a big waterfall, or raindrops tapping on your window. Water can be peaceful but also harmful. It all lies in the physics behind it. In this section we’re diving into the cool stuff about water—how it moves, how heavy or light it is, the strong pushes it can give, and even its colors. Get ready for a splash of fun as we explore the secrets of water. It’s time for a watery adventure! 🌊💦
1. Phase Transitions
Water can be observed in nature in three forms: Liquid, ice and gas. The transitions between water’s three phases are not only changes in state but also involve dynamic energy exchanges. For example, during freezing, the release of latent heat creates amazing ice crystal structures, contributing to the diversity of snowflakes. Understanding these phase transitions is crucial for predicting weather patterns and studying the cryosphere. The term cryosphere refers to the frozen parts of the planet. It includes snow and ice on land, ice caps, glaciers, permafrost, and sea ice. This sphere helps maintain Earth’s climate by reflecting incoming solar radiation back into space.
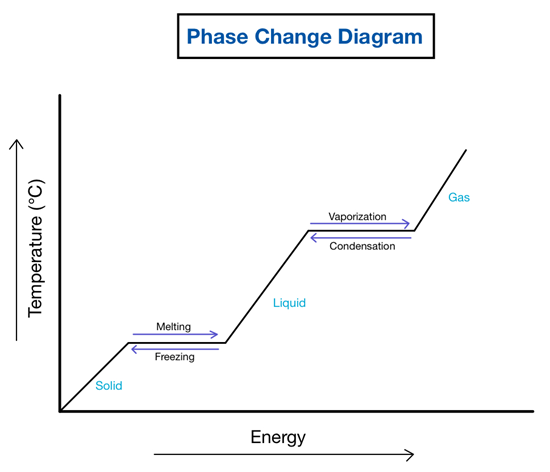
The diagram shows that it is not possible for ice to become gas directly. However, we will see examples in real life where this occurs: Yes, ice can transform directly into a gas through a process called sublimation. Sublimation occurs when a substance transitions from the solid phase (ice) to the gaseous phase (water vapour) without passing through the intermediate liquid phase.
In the case of water, if the surrounding conditions, such as temperature and pressure, allow it, ice can sublimate directly into water vapor. This process is the reverse of deposition, where water vapor transitions directly into ice.
An everyday example of sublimation is the way frost on frozen foods in a freezer disappears over time without melting into a liquid. The frost undergoes sublimation, turning directly from solid (ice crystals) into water vapour.
2. Density
Density is a measure of how much mass is packed into a given volume. For water, its density is influenced by its molecular structure and temperature. The standard density of pure water is typically considered to be 1 gram per cubic centimeter (g/cm³) at 4 degrees Celsius (39.2 degrees Fahrenheit).
<How much liter is 1kg of water?>
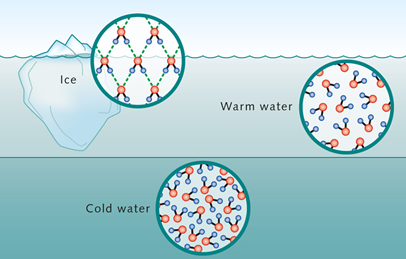
Since ice is water what makes it denser? Does the arctic ice float on top of the water? This characteristic lies in the fact that the hexagonal arrangement of molecules in the solid lattice when forming ice. This property is vital for aquatic ecosystems as ice floats, insulating the water below and allowing life to thrive in sub-zero temperatures.
This difference in molecular structure can also be observed with cold and hot water. Hot water is less dense and hence floats on cold water. This characteristic has macro effects in the currents in our oceans
3. Hydrostatic Pressure
Hydrostatic pressure, a result of the column of water above a specific point, is a fundamental concept in oceanography and geophysics. The pressure gradient influences ocean circulation patterns and plays a role in the formation of deep-sea trenches. Exploring these pressure variations provides insights into the dynamics of underwater ecosystems.
Hydrostatic pressure refers to the pressure exerted by a fluid (such as water) at rest due to the force of gravity. This pressure is uniform at a given depth in a fluid and acts in all directions. The deeper you go underwater, the greater the hydrostatic pressure.
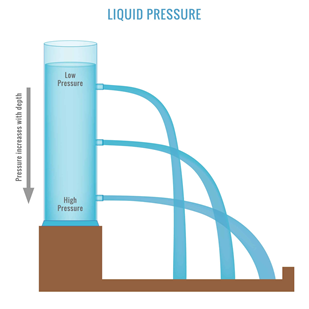
Hydrostatic pressure has lots of meaning in our everyday lives. From pipes to water basins to water towers. It is important to be aware of this principle when talking about water. An easy representation of hydrostatic pressure is that the more mass that is present on top creates more pressure at a certain depth.
4. Hydrodynamics
The study of hydrodynamics encompasses the motion of water and its interaction with surrounding elements. Viscosity, a measure of a fluid’s resistance to flow, influences the speed and patterns of rivers and ocean currents. Turbulence, an intricate dance of chaotic flows, shapes coastlines and contributes to the dispersion of nutrients and heat in aquatic environments.
Hydrodynamics might sound like a big word, but let’s break it down in a super simple way. Imagine you’re at the pool, and you see the water moving when someone jumps in or when you splash around. That movement of water, how it flows and swirls, is what hydrodynamics is all about.
Here are a few cool things about hydrodynamics:
- Water Dance: Picture a stream or river. Hydrodynamics helps us understand why the water flows smoothly in some parts and gets a bit wild in others. It’s like a dance of water!
- Boat Magic: Ever wondered why boats can float and move? Hydrodynamics is the reason! It helps engineers design boats that glide through the water with ease.
- Hidden Forces: When you stir your drink with a straw, or when you see leaves floating in a pond, hydrodynamics is at play. It’s like a secret force making things move in water.
- Water Tricks: Hydrodynamics helps scientists figure out how waves work at the beach or why raindrops fall the way they do. It’s like solving puzzles about water tricks!
So, hydrodynamics is basically the science that helps us understand how water moves, why it does cool things, and how we can use this knowledge for boats, waves, and all sorts of watery wonders. Even when calculating how to clean rivers from plastics we have to use hydrodynamics to predict the flow of trash and point of accumulation. Isn’t it cool?! 🌊✨